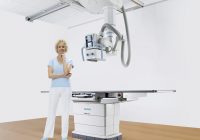
Siemens Ysio Max Digital Radiography System FDA Cleared
Siemens Healthineers won clearance from the FDA for the Ysio Max digital radiography system that features a number of so-called MAX technologies, including new detectors and usability features that improve imaging and quicken exams. Three new detectors are included, including the new MAX wi-D, the lightest 14 x 17” wireless detector with a handle, that… Read More »